PARTNERS IN
Oncology, Pathology and Diagnostics
Being ready for changes in healthcare delivery and personalized medicine will help improve competitiveness in a market where large reference laboratories are vying for patient samples, hospitals are consolidating physician practices, diagnostic centers and other outsourced services, and diagnostic manufacturers are offering laboratory services for their own commercial products.
TECHNOLOGY FROM TOMORROW
Pathology, Oncology & Technical Services
- Technical or professional service partner
- On-site laboratory management for flow cytometry
- Molecular diagnostic startup support
- Test menu assessment
- Technology & platform assessment
- Testing implementation support
- Technical training and validation
- Mock inspections for CAP compliance
- IT software and support

GENEUITY CRS
Clinical Research Services
Geneuity Clinical Research Services has partnered with the clinical and research community for a decade. We are a leader in the integration of molecular diagnostics and pathology testing for pharma, clinical research organizations, and medical device companies. We are experienced in outsourced specialty testing and device validation and our expert clinical and scientific staff provide a consultative approach to protocol review and biomarker selection.
- Companion diagnostic reference laboratory commercial partner
- Companion diagnostic validation
- Biomarker assay assessment
- IVD data collection
- Patient recruitment screening
By the numbers
Sed imperdiet, ante in porta ultrices, orci metus consequat lacus, ac viverra magna odio at dui. Sed id congue velit, nec gravida urna. In vestibulum nibh magna, at dapibus est molestie vulputate.
2300Clients
47MVisitors
150BUSD Profit
550Locations
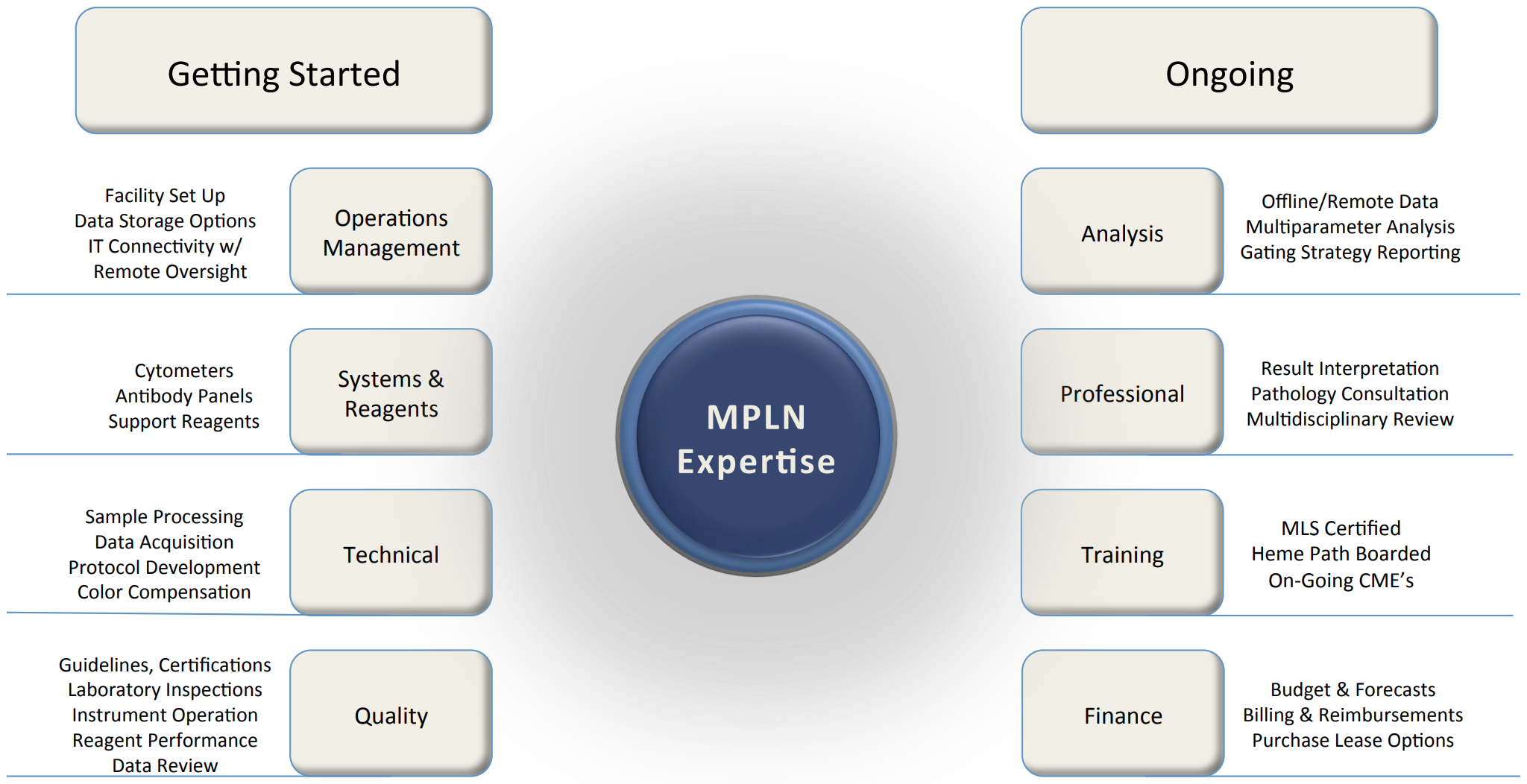
Partners in Flow Cytometry
Experience MPLN’s Expertise
With more than two decades experience, Molecular Pathology Laboratory Network, Inc., (MPLN) can offer our vast resources and knowledge toward getting started in flow cytometry; we provide consultation and laboratory management options; we can also work with your existing infrastructure and personnel or provide total laboratory operation.
Partner with MPLN and experience the difference expertise brings.
Guideline and Certification Resources:
- . 2006 Bethesda recommendations on flow cytometry immunphenotypic analysis of hematolymphoid neoplasia-medical indications; training and education; optimal reagents and reporting. Cytometry B Clin. Cytom 72 S1.
- Qualification-in-Cytometry, QCYM. ASCP Board of Certification.
Flow Cytometry Services
Thinking about setting up your own flow cytometry facility? What are the benefits to you and your patients, can you provide a cost efficient operation, and how much training is required? Consider the following:
- Provide better service to your patients
- Deliver faster turnaround time on results
- Retain more reimbursement dollars, operate at a lower cost
- Perform technical and/or professional component
- Provide training and career path for your medical technologists
Providing a comprehensive service may seem straight forward, but the challenge is immediate and ongoing. Unlike routine hematology, flow cytometry still requires much intervention. Your results are only as good as your flow cytometry operator. Training and continuous education are pre-requisites to a competent flow cytometry operation. Only with an experienced cytometry operator can you start up and run a flow cytometry operation out the box.